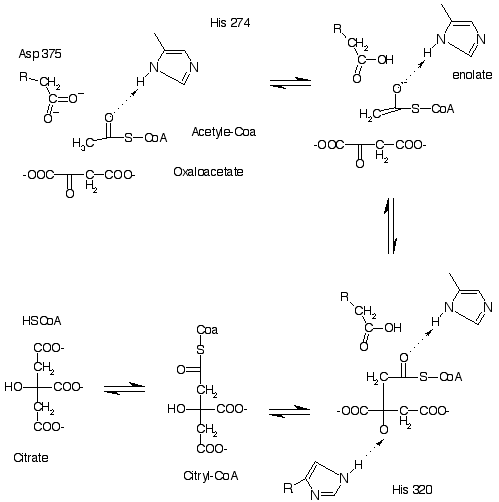
Citrate synthase catalyzes the condensation of oxalacetat and acetylcoA into citrylcoA, which is then hydrolyzed to citrate and coA. The mechanism of the reaction proceeds according to three steps: enolization, condensation and hydrolysys. There are some residues in the active site, conserved in a large number of species, that have an important role in the catalysys.These crucial residues are: two histidines and an aspartic acid. The some of the surrounding residues are also involved in the catalysis but their contribution is not fully understood.
Histidine 274, Histidine 320, and Aspartic Acid 375 in the active site act to hold oxaloacetate and acetyl coA, once the substrtates are bound the enzyme adopts the proper conformation for catalysis. Each residue has an inherent function in the active site:
- His 320 holds the carbonyl of oxaloacetate, the alcohols of Citryl-coA, and the citrate through a hydrogen bond interaction.
- Asp 375 is in the acetyl coA binding domain which brings about the formation of an enolate intermediate.
- His 274 forms two different bonds, stabilizing both the thioester carbonyl of acetyl coA and the carboxylate of citrate after hydrolysis of the thioester intermediate.
The stability of the reaction resides in these critical amino acids and their conformation, bringing about the production of citrate. Detailed steps of the global reaction are:
ENOLIZATION A neutral enol intermediate is brought about mainly by the abstraction of a proton from the methyl group of acetyl coA by acid-base catalysis. His 274 donates a proton to the carbonyl oxygen of the acetyl group, while a proton from the methyl group is simultaneous accepted by Asp 375. This neutral molecule bypasses the production of either enolate or ketone groups (ketoenolic equilibium) which both would be energetically very high. This step requires Asp 375 to be negatively charged while His 274 remain protonated and positively charged.CONDENSATION After the neutral enol has been generated, His 320 protonates the carbonyl oxygen of oxaloacetate. Through the guidance of His 274 the second carbon of the enol attacks the carbonyl carbon of oxaloacetate thus forming the intermediate in the reaction: citryl-coA. So the carbonyl carbon of oxaoacetat is attacked by the neutral enol intermediate to form C-C bond. HYDROLYSYS The newly formed citriylcoA induces additional structural changes in the enzyme. The active site becomes completely enclosed, and Aspartate residue is recruited to enable a trapped water molecule. This molecule attacks the carbon of the citryl to coA bond (thioester bond), hydrolyzing it, and causing the release of both coA and citrate product. |
 |
Two conformations of the CS enzyme have been determined: opened and closed . The open conformation exists when the active site is not occupied and is thus free to bind substrate molecules for enzyme catalysis. The closed conformation is believed to exist during catalysis. A structural shift causes the movement from the open to the closed conformation, visible in a 18 degree rotation of the small domain relative to the large domain. During catalysis, conformational changes bring about the closing of citrate synthase like the closing of a thumb and forefinger. The open form of citrate synthase leaves the active site open to interact with solvent, while the closed form with CoA to the outside of the enzyme isolates the active site for catalysis .
This confomational shift is absolutely necessary because if the active site is open to interaction with solvent, the intermediates of the reaction would either not form or would be too unstable. Thus because of this exclusion of solvent, and inclusion of substrates, catalysis occurs.