IVYA
The human
'protective protein' (HPP) forms a multi-enzyme complex with ![]() -galactosidase
and neuraminidase in the lysosomes, protecting these two glycosidases from
degradation. In humans, deficiency of HPP leads to the lysosomal storage
disease galactosialidosis: a lysosomal storage disease inherited as an
autosomal recessive trait. Patients with this disorder are biochemically
diagnosed as having drastically reduced
-galactosidase
and neuraminidase in the lysosomes, protecting these two glycosidases from
degradation. In humans, deficiency of HPP leads to the lysosomal storage
disease galactosialidosis: a lysosomal storage disease inherited as an
autosomal recessive trait. Patients with this disorder are biochemically
diagnosed as having drastically reduced ![]() -galactosidase
and neuraminidase activities in the lysosomes. Slightly different spectra of
symptoms are observed : skeletal dysplasia, dysmorphism, progressive
neurological deterioration, reduced life expectancy, and in some cases mental retardation,
as well as impairment of cardiac and kidney function.
-galactosidase
and neuraminidase activities in the lysosomes. Slightly different spectra of
symptoms are observed : skeletal dysplasia, dysmorphism, progressive
neurological deterioration, reduced life expectancy, and in some cases mental retardation,
as well as impairment of cardiac and kidney function.
The activation mechanism of HPP is unique among proteases with known
structure. It differs from the serine proteases in that the active site is
preformed in the zymogen, but is blocked by a maturation subdomain. In contrast
to the zinc metalloproteases and aspartic proteases, the chain segment
physically rendering the catalytic triad solvent inaccessible in HPP is not
cleaved off to form the active enzyme. The activation must be a multi-step
process involving removal of the excision peptide and major conformational
changes of the maturation subdomain, whereas the conformation of the enzymatic
machinery is probably almost, or completely, unaffected.
HPP is synthesized as a 542 amino acid precursor with a molecular weight
of 54 kDa and dimerizes soon after synthesis in the endoplasmic reticulum. The
sequence contains two glycosylation sites (Asn117 and Asn305) and nine
cysteines. After transport to the acidic endosomal/lysosomal compartments, the
precursor undergoes a protease-mediated maturation process. A polypeptide of
approximately 2 kDa, called the 'excision' peptide is removed from the protein
yielding a 32 kDa and a 20 kDa chain held together by disulfide bridges
The crystal
structure of the 108 kDa dimer of the precursor HPP has been elucidated by
making extensive use of twofold density averaging. The monomer consists of a
'core' domain and a 'cap' domain.
HPP appears to be a multi-functional lysosomal enzyme.
Although the exact physiological substrate(s) of HPP are not known, there is
evidence that HPP may be secreted to participate extracellularly in the
deactivation of selected bio-active peptides such as endothelin I. While loss
of the protective capacity of HPP is directly linked to galactosialidosis, it
is unclear to what extent loss of the enzymatic activity contributes to the
clinical phenotype. Given its pleiotropic and distinct functions, as well as
the capacity to be secreted and taken up by different cell types, HPP may have a
function outside the multi-enzyme complex and possibly outside the lysosomes as
well.
The crystal
structure determination of the precursor form of HPP shows that the monomer contains approximately 20% ![]() structure
and 30% helical structure. The protein fold can be divided into two domains: a
'core' domain, as commonly found in members of the hydrolase fold family and a
'cap' domain. The core domain, comprising residues 1–182 as well as 303–452,
contains a central ten-stranded
structure
and 30% helical structure. The protein fold can be divided into two domains: a
'core' domain, as commonly found in members of the hydrolase fold family and a
'cap' domain. The core domain, comprising residues 1–182 as well as 303–452,
contains a central ten-stranded ![]() sheet.
An additional ten
sheet.
An additional ten ![]() helices
and two small
helices
and two small ![]() strands
occur on both sides of the central
strands
occur on both sides of the central ![]() sheet.
The cap domain can be divided into a 'helical' subdomain consisting of three
sheet.
The cap domain can be divided into a 'helical' subdomain consisting of three ![]() helices
(residues 183–253) and a 'maturation' subdomain consisting of a three-stranded
mixed
helices
(residues 183–253) and a 'maturation' subdomain consisting of a three-stranded
mixed ![]() sheet
(residues 254–302).
sheet
(residues 254–302).
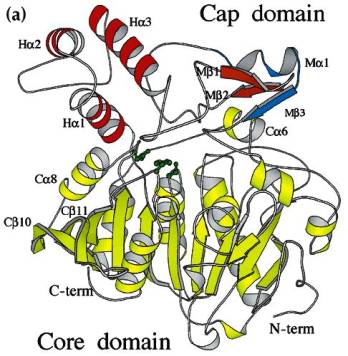
Fig. 1.Schematic ribbon diagram of
the HPP monomer (monomer 1). The 'core' domain is shown in yellow. The 'cap'
domain consists of a 'helical' subdomain (red) and a 'maturation' subdomain
(orange). The 'excision' peptide, located in the maturation subdomain is shown
in light blue. The side chains of the catalytic triad Ser150, His429 and Asp372
(from right to left) are in green. Some secondary structure elements (assigned
according to DSSP) are labeled.
There appear to be four disulfide bridges per monomer:
Cys60–Cys334, Cys253–Cys303, Cys212–Cys228 and Cys213–Cys218.
HPP (only
its mature form) possesses serine carboxypeptidase activity at acidic pH. Based
on sequence alignments with serine carboxypeptidases, the catalytic triad
in HPP has been proposed to be formed by the residues Ser150, His429 and
Asp372 members of the hydrolase fold family, which includes enzymes with
different catalytic functions such as the serine carboxypeptidases,
dehalogenase, various lipases and acetylcholinesterase. Although the central
core is the same (a central ![]() sheet
flanked by
sheet
flanked by ![]() helices
on both sides), the cap domains in this protein family are quite diverse, both
with respect to their folds as well as their sizes. HPP has one of the largest
cap domains, with 121 residues forming the three-helix bundle of the helical
subdomain and the three-stranded
helices
on both sides), the cap domains in this protein family are quite diverse, both
with respect to their folds as well as their sizes. HPP has one of the largest
cap domains, with 121 residues forming the three-helix bundle of the helical
subdomain and the three-stranded ![]() sheet
of the maturation subdomain.
sheet
of the maturation subdomain.
The oxyanion hole proposed to stabilize the negatively charged
tetrahedral intermediate in serine carboxypeptidases is formed in HPP by the
backbone amides of Gly57 and Tyr151.
In HPP, a
pair of glutamic acid residues (Glu69 and Glu149) is positioned near the
catalytic triad, with their carboxylate groups interacting with each other. In
addition, an asparagin (Asn55) is oriented such that it forms a hydrogen
bond to each of the two carboxylate groups of the glutamic acid pair. These
three residues are conserved between HPP, CPW and CPY and have been implicated
in regulating the low pH optimum for the carboxypeptidase activity found
in the serine carboxypeptidases
HPP has a substrate preference for hydrophobic
residues in the P1 and/or P1' binding pockets. The P1' pocket was
identified as consisting of Tyr247 and Asp64, with Met430 and Thr304 at
the far end.
The active-site cleft in the precursor is blocked by
numerous residues from the maturation subdomain. The catalytic triad is
rendered solvent inaccessible by residues Asn275, IIe276 and Phe277. These
residues are part of the polypeptide Asp272–Phe277 which we call the 'blocking'
peptide. This peptide is held down predominantly by hydrophobic contacts.
Residue Asn275 of the blocking peptide appears to fill what might be part of
the P1 binding pocket in the mature form. The blocking peptide does not assume
a conformation that a productive peptide substrate would adopt. It is carefully
positioned to avoid being cleaved by the nearby catalytic residues. Thus,
substrate binding is prevented in the precursor form by the inaccessibility of
the substrate-binding pockets. Our structure reveals that the inactivation
mechanism of HPP is based on blocking of the active site, not upon conformational
changes of the residues involved in catalysis or transition-state
stabilization.But maturation must be accompanied by conformational changes.
In HPP a
fourth mechanism for protease zymogen activation is revealed. In this case, the
catalytic triad in the precursor form is in a catalytically competent
conformation. Enzymatic activity is prevented by the blocking peptide. The
blocking peptide is, however, not the same as the excision peptide. This leads
to a distinct difference from other maturation mechanisms. After disappearance
of the excision peptide, up to 35 residues filling the active-site cleft in the
HPP precursor must rearrange to render the catalytic triad solvent accessible,
but these residues do not get cleaved off. Removal of the excision peptide, and
possibly a shift to lower pH in the endosome/lysosome, appears to be a trigger
for this event.