The ability of the body to control the flow of blood following vascular injury is paramount to continued survival. The process of blood clotting and then the subsequent dissolution of the clot, following repair of the injured tissue, is termed hemostasis. Hemostasis is composed of 3 major events:
Primary hemostais
The initial phase of the process is vascular constriction. This limits the flow of blood to the area of injury.
Secondary hemostasis
Next, starts the coagulation cascade. It is a complex process by which blood forms clots. It is an important part of hemostasis (the cessation of blood loss from a damaged vessel), wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel. Disorders of coagulation can lead to an increased risk of bleeding (hemorrage) or clotting (thrombosis).
Tertiary hemostasis
Finally, the clot must be dissolved in order for normal blood flow to resume following tissue repair. The dissolution of the clot occurs through the action of plasmin . This process is called fibrinolysis.
The coagulation cascade
Two pathways lead to the formation of a fibrin clot: the intrinsic and the extrinsic pathway. The extrinsic pathway is the response to tissue injury, meanwhile the intrinsic pathway is the response to an abnormal vessel wall. Although they are initiated by distinct mechanisms, the two converge on a common pathway that leads to clot formation.
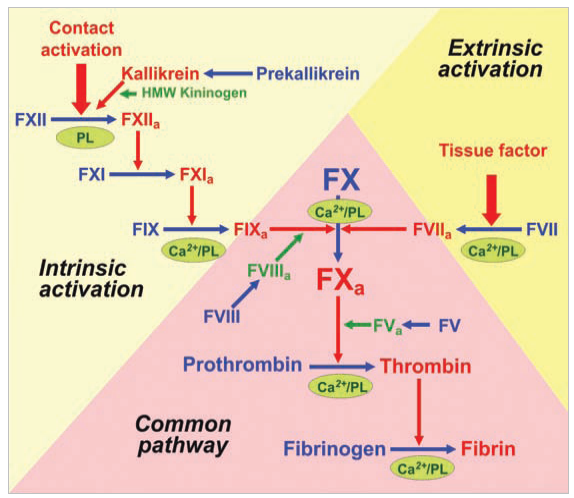
It's a cascade
The fact that the coagulation process is performed as a cascade can not be overlooked. It has advantages of its own. The multiple steps of the cascade amplify the signal from that first stimulus. If a single active molecule of Factor XII could activate, say, 20 or 30 molecules of Factor XI, then each level of the cascade would multiply the effects of a starting signal. Putting 5 or 6 steps in the cascade, the biochemical signal will be amplified more than a million times. Clotting with fewer steps would still work, but it would take longer to produce a substantial clot, and would be much less responsive to smaller injuries.
Both pathways are complex and involve numerous different proteins termed clotting factors.
Factor I
(Fibrinogen)
|
Cleaved by thrombin to form fibrin clot
|
Factor II
(Prothrombin)
|
Activated on surface of activated platelets by prothrombinase complex
|
|
Factor VII
|
Activated by thrombin in presence of Ca2+
|
|
Factor X
|
Activated on surface of activated platelets by tenase complex and by factor VIIa in presence of tissue factor and Ca2+
|
|
Factor IX
|
Activated by factor XIa in presence of Ca2+
|
|
Factor XI
|
Activated by factor XIIa
|
|
Factor XII
|
Binds to exposed collagen at site of vessel wall injury, activated by high-MW kininogen and kallikrein
|
|
Factor XIII
|
Activated by thrombin in presence of Ca2+; stabilizes fibrin clot by covalent cross-linking
|
In addition, some proteins, called cofactors, are required for the proper functioning of the coagulation cascade. And, also, there are other regulatory proteins needed to keep platelet activation and the coagulation cascade in check.
|
Cofactors
|
Factor VIII
|
Activated by thrombin; factor VIIIa is a cofactor in the activation of factor X by factor IXa
|
|
Factor V
|
Activated by thrombin; factor Va is a cofactor in the activation of prothrombin by factor Xa
|
Factor III
Tissue factor
|
Subendothelial cell-surface glycoprotein that acts as a cofactor for factor VII
|
|
Regulatory elements
|
von Willebrand factor
|
Associated with subendothelial connective tissue; serves as a bridge between platelet, glycoprotein GPIb/IX and collagen
|
|
Protein C
|
Activated to protein Ca by thrombin bound to thrombomodulin; then degrades factors VIIIa and Va
|
|
Protein S
|
Acts as a cofactor of protein C; both proteins contain gla residues
|
|
Thrombomodulin
|
Protein on the surface of endothelial cells; binds thrombin, which then activates protein C
|
|
Antithrombin III
|
Most important coagulation inhibitor, controls activities of thrombin, and factors IXa, Xa, XIa and XIIa
|
What is interesting on the factors involved in the coagulation cascade is that all of them are trypsin-like serine proteases and all of them come from a common ancestor in the very far past, the trypsin. And what makes this proteins more interesting is that they, now, have functions involved in the same process and that their structure is quite similar. But, at the same time, they are distinct enough to be responsible of a specific step inside the delicate and absolutly necessary process of the coagulation.
We will now introduce a subset of all the serine proteases of the coagulation that will allow us to illustrate the relationship of that proteins.
Zimogens ... what?
A zimogen is an inactivated enzyme precursor in which are synthesized many enzymes. It needs to be activated in order to be an active enzyme able to get involved in the catalysis of reactions.
Proteolytic enzymes are synthesized as zymogens to prevent unwanted protein degradation, and to enable spatial and temporal regulation of proteolytic activity. Upon sorting or appropriate compartmentalization, zymogen conversion to the active enzyme typically involves limited proteolysis and removal of an "activation segment." The sizes of activation segments range from dipeptide units to independently folding domains comprising more than 100 residues. A common form of the activation segment is an N-terminal extension of the mature enzyme, or "prosegment," that sterically blocks the active site, and thereby prevents binding of substrates. In addition to their inhibitory role, prosegments are frequently important for the folding, stability, and/or intracellular sorting of the zymogen.
The inactive serine proteases are zimogens and need to be cleaved in order to be activated. The elements that cleave them are, in general terms, other serine proteases that, equally, are zimogens that have had to be activated by being cleaved by other serine proteases.
