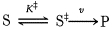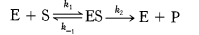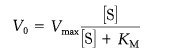Enzymes are the catalysts of biological systems.They determine the patterns of chemical reactions,thanks to their catylitic power and specificity,that takes place in the part of the enzyme called active site.
They catalyze reactions by stabilizing transition states, the highest-energy species in reaction pathways. By selectively stabilizing a transition state, an enzyme determines which one of several potential chemical reactions actually takes place.
Enzymes can be classified in 6 majors classes:
-
Oxidoreductases->Oxidation-reduction E.g.Lactate dehydrogenase
-
Transferases->Group transfer E.g.Nucleoside monophosphate kinase
-
Hydrolases ->Hydrolysis reactions E.g.Chymotrypsin
-
Lyases ->Addition or removal of groups to form double bonds E.g.Fumarase
-
Isomerases ->Isomerization (intramolecular group transfer) E.g.Triose phosphate isomerase
-
Ligases -> Ligation of two substrates at the expense of ATP hydrolysis E.g.Aminoacyl-tRNA synthetase
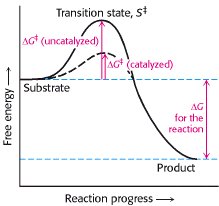
The chemical reaction of substrate to product goes throug a TRANSITION STATE. The difference in free energy between the transition state and the substrate is called the Gibbs free energy of activation or simply the activation energy.
Enzymes facilitate the formation of the transition state lowering the activation energy,without altering the Energie gradient of the reaction.
Enzymes accelerate reactions by decreasing the activation energy.
Linus Pauling
"I think that enzymes are molecules that are complementary in structure to the activated complexes of the reactions that they catalyze, that is, to the molecular configuration that is intermediate between the reacting substances and the products of reaction for these catalyzed processes. The attraction of the enzyme molecule for the activated complex would thus lead to a decrease in its energy and hence to a decrease in the energy of activation of the reaction and to an increase in the rate of reaction."
Linus Pauling Nature 161(1948):707

Linus Carl Pauling (February 28,1901-August 19,1994)
He has been awarded a Nobel Prize in two different fields (the Chemistry and Peace prizes).
ACTIVE SITE
The active site of an enzyme is the region that binds the substrate. The interaction of the enzyme and substrate at the active site promotes the formation of the transition state.
Some carachteristics of the active site:
-
The active site is a three-dimensional cleft or crevices formed by groups that come from different parts of the amino acidsequence.
-
The specificity of binding depends on the precisely defined arrangement of atoms in an active site.
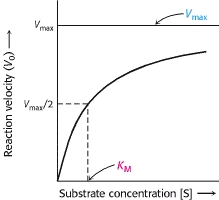
The Michaelis-Menten model accounts for the kinetic properties of some enzymes.In this model, an enzyme (E) combines with a substrate (S) to form an enzyme-substrate (ES) complex, which can proceed to form a product (P) or to dissociate into E and S.
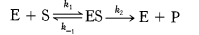
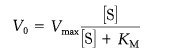
The model is used to evaluate the efficiency of the differents enzymes.